CHEMISTRY :
Chromatography
RF Values
Background
As described in the main chapter of this section, in paper chromatography there is what is known as the stationary phase which is the absorbent Chromatography paper and the mobile phase which is a liquid solvent (or mixture of solvents) used to carry the sample solutes under analysis along the paper. Usually, one uses chromatography to find out the components of a sample which are seperated depending how much soluble these are in particular solvents and hence how far they travel along the chromatography paper. Samples are usually of organic matter (not ionic salts) which dissolve in certain polar solvents (namely water) or non-polar (organic) solvents.
Principle
In order to make the technique more scientific rather than a mere interpretation by sight, what is called the Retention Value (Rf value for short) was applied in chromatography. A particular compound will travel the same distance along the stationary phase by a specific solvent (or solvent mixture) given that other experimental conditions are kept constant. In other words, every compound (dye, pigment, organic substance etc) have a specific Rf value for every specific solvent and solvent concentration. Rf values come very handy for identification because one can compare Rf values of the unknown sample (or its consituents) with Rf Values of known compounds.
Calculation
The Rf value is defined as the ratio of the distance moved by the solute (i.e. the dye or pigment under test) and the distance moved by the the solvent (known as the Solvent front) along the paper, where both distances are measured from the common Origin or Application Baseline, that is the point where the sample is initially spotted on the paper.
| Rf Value = |
Distance from Baseline travelled by Solute |
| |
|
| |
Distance from Baseline travelled by Solvent (Solvent Front) |
Due the fact that the solvent front is always larger from the distance travelled by the solute, Rf values are always between 0 - one extreme where solute remains fixed at its origin and 1 - the other extreme where the solute is so soluble that it moves as far as the solvent. Rf values do not have units since it is a ration of distances. Because mixture solvents are often applied Rf values are usually written as the following examples:
- Rf = 0.66 (60% Ethanol) - if % is given it is assumed that the mixture is in water hence 60% ethanol 40% water.
- Rf = 0.78 (Ethanol-Methanol mixture {1:2}) - a mixture of 1 part Ethanol and 2 parts Methanol
- Rf = 0.25 (Ethanol-Methanoic Acid-Acetone mixture {4:3:1}) - a mixture of 4 parts Ethanol, 3 parts Methanoic Acid and 1 part Acetone. Note that mixture compounds with larger proportions are placed first in the mixture sequence.
Rf Values for Identification
Note that different componds can have the SAME Rf value for a particular solvent, but unlikely to have similar Rf for a number (2-4) of different solvents. Therefore the more different solvents (or mixtures) are used, the more RF values are obtained, and so the more concise the identification is. Identification relies on comparing a number of RF values of the unknown sample with known Rf values of a number of known dyes.
Environment Conditions
As mentioned before, the Rf value of a particular pure dye or analyte in a particular solvent (or mixture) is constant if the following experimental conditions are kept unaltered:
- Temperature
- Chromatography medium, ie same type and grade of Chromatography Paper
- Solvent concentration and purity
- Amount of sample spotted on Chromatography medium
If the same grade of Chromatography medium is used (typically Grade 1 CHR or 3 MM CHR) and the room temperature of the experiment does not fluctuate too much, the remaining critical variable to be observed is the amount of dye spotted. Large amounts tend to form elongated zones with uneven distribution of dye along its zone. Too much dilute spots makes visibility of seperated dye poor. Trial and error is involved to find the ideal proximate amount to be spotted.
Problems with dye zones so as to determine Rf Values
In the ideal scenario, the zone of the dye or component moved along the chromatography paper is a small, compact disc-like structure. In the real world, the zones can be elongated (streak-like) and this brings the problem of where should one take the measurment to caclulate the Rf value - either taken from the top, or the centre or the bottom of the zone! Actually the zone length can vary from 4 to 40 mm. By definition, the Rf value is taken as the distance from the centre of te zone. This is however prone to visual estimation errors, so the best way to calculate the centre is to measure the following 2 distances:
- measurment from origin to the top edge of the zone,
- measurment from origin to the bottom edge of the zone
The measurment of the central part is then determined by average of the top and bottom edge distances , hence (Distance_top + Distance_bottom / 2). In these docs, I also measure the Rf value from the top edge of the zone and therefore you would find two Rf values per analyte:
(a) Rf Top)and
(b) Rf center
The diagram below explains what are the distances to be taken to calculate these RFs
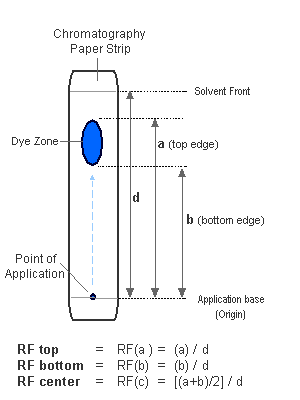
By defenition the actual RF is that of the center (b) but all three should be compared and analysed. Shorter, compact zones give more accurate results, while elongated streak-like zones (especially starting from the origin) should be discarded as in such cases, the Rf values are not reliable. Zones with uneven distibution of dye or atypical shapes should also be discarded and RF value in other solvents with good zones should be seeked. The reference RF Value should be calculated from at least 3 different runs.
Specific Rf Values of Dyes and compounds obtained in the Lab
Below are the RF value results obtained from various, either known ones or those isolated from inks, markers etc. Click on dye name to see the table of results. Note that the smaller the standard deviation is, the more accurate are the results. The method is standardised as much as possible to provide reproducible and reliable results. Follow the method in this link here
| Category |
In-house
Name |
Scientific
Name |
Rf (centre) |
Rf (top) |
Table of Results |
| Ink |
Fast Violet 01 |
Methyl Violet ? |
0.90 (60% Ethanol)
0.84 (60% Propanone) |
0.95 (60% Ethanol)
0.91 (60% Propanone) |
Click Here |
| Ink |
Sky Blue 01 |
Unidentified |
0.78 (60% Ethanol)
0.65 (60% Propanone) |
0.84 (60% Ethanol)
0.75 (60% Propanone) |
Click Here |
| Ink |
Light Blue FVT 01 |
Unidentified |
x (60% Ethanol)
x (60% Propanone) |
x (60% Ethanol)
x (60% Propanone) |
not available |
Chromatography Section Links :
[ Main Page ] -
[ Methodology ] -
[ Solvents Used ] -
[ Rf Values ] -
[ Ink Sample Analysis ] -
[ Extracted Dyes ] -
[ History ]
Chemistry Section Links
[ Chemistry Main Page ] -
[ Qualitative & Quantitative Analysis ] -
[ Pyrotechnics ]
[ Cation ID ] -
[ Chromatography ] -
[ Crystal Study ] -
[ Misc Documents ] -
[ Chemistry Index Page ]


